MSK-IF Project Process
MSK-IF follows a streamlined co-design process to develop user-driven digital health solutions that solve real-world MSK health and mobility problems. This process is rooted in the conception, development, and delivery of projects with immediate clinical applicability. Our user-centric approach ensures effective project management, promotes ongoing assessment and encourages continuous improvement.
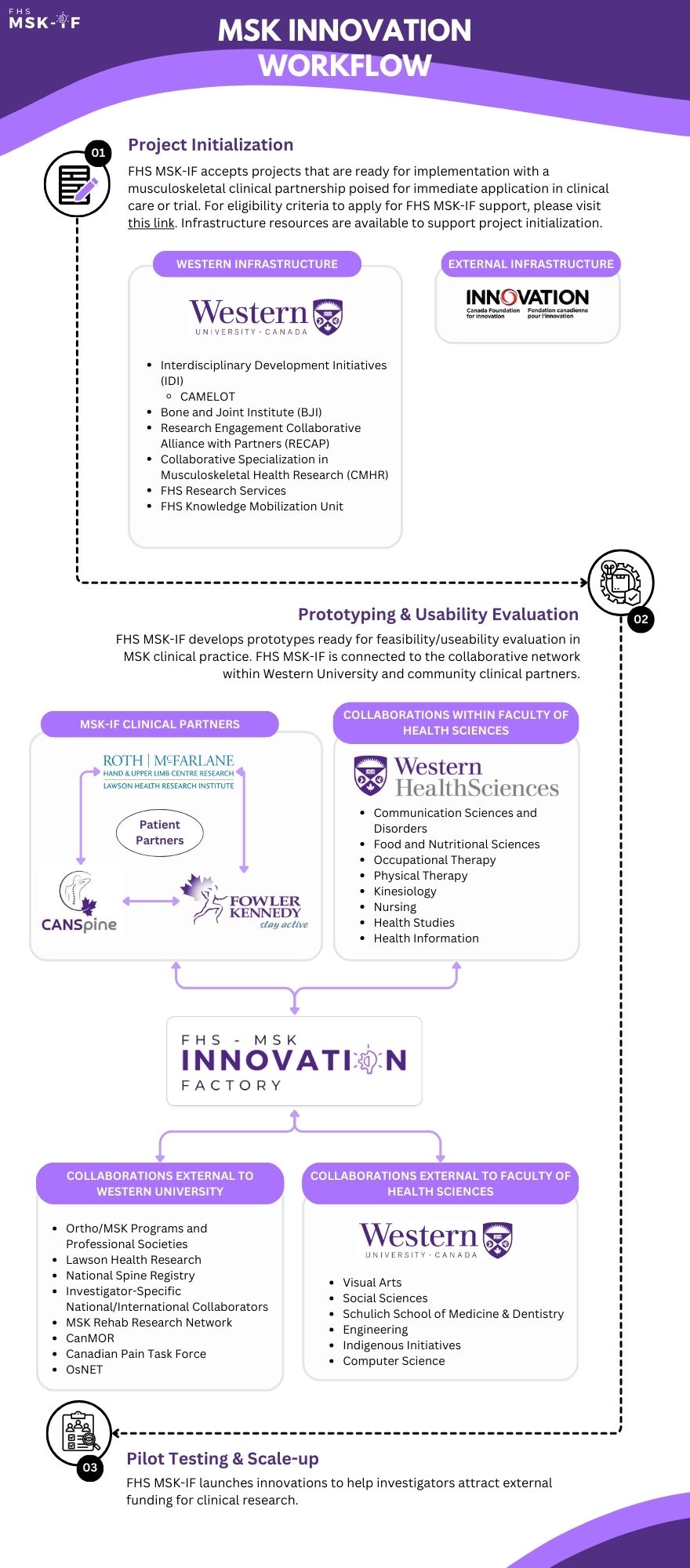
We accept projects that are ready for implementation and possess a clinical partnership poised for immediate application in clinical care or trial. To propose a project to MSK-IF, please review the following eligibility criteria, submit a Letter of Intent (sample) and forward it to mskif@uwo.ca.
Eligibility Criteria- The principal investigator is a researcher within the Faculty of Health Sciences at Western University
- The project aims to develop an innovative solution to musculoskeletal health issues
- The project/idea has been co-developed with patient/clinical partners and is poised for immediate application in clinical research or implementation in practice
Stage 1. Initiation

The principal investigator (PI) interested in MSK-IF involvement submits a Letter of Intent (LOI) to the MSK-IF, which provides an overview of the project, its relevance to MSK health, and what they seek from MSK-IF. The LOI is initially screened by MSK-IF Operations Committee to assess its alignment within the MSK-IF scope. If deemed appropriate, the PI is asked to submit this proposal form to MSK-IF Adjudication Committee for approval for development.
Stage 2. Planning

A comprehensive project plan is developed during this phase. This plan breaks down the project into specific measurable tasks and establishes timelines and milestones for each. Resources are allocated, potential risks are identified, and mitigation strategies are developed. This stage is critical for setting the path for the project and providing a roadmap.
Stage 3. Execution

The execution phase involves carrying out the tasks laid out in the project plan. The progress is regularly monitored and compared to the project plan to manage development effectively. The project iteratively receives clinical & patient partners' feedback to ensure that the project stays relevant to user needs. Necessary adjustments are made in response to any unforeseen circumstances, ensuring the project stays on track.
Stage 4. Closure

The closure phase marks the completion of the project. It involves wrapping up all project tasks, archiving project materials, and conducting a project retrospective.

